Pressure & Diving
Pressure is any force applied to an object. When you press on an object, you're applying pressure to it. Your car's tires are kept firm by the pressure of compressed air pushing out on the inside. Scales measure weight by reading the increase of pressure on top of it. As divers, we measure pressure to determine how full our cylinders are and how deep we are.
Pressure is also the cause of many diving injuries. Ear squeezes, lung overexpansion, and decompression sickness are just a few examples of injuries caused by pressure changes. For this reason, it's important to understand pressure and how it affects air spaces.
In this lesson you'll learn about atmospheric pressure, pressure underwater, and how pressure changes affect the volume and density of air.

Atmospheric Pressure
The weight of the air above us exerts pressure, and this is called atmospheric pressure.
Exactly how much pressure is exerted on us depends on altitude. Pressure is lowest at high altitudes because there is less air above you. And at lower altitudes, there is more air above you, so pressure increases.
In the United States, we use pounds per square inch (psi) to measure pressure. PSI refers to the amount of pressure exerted on 1 square inch of a surface.
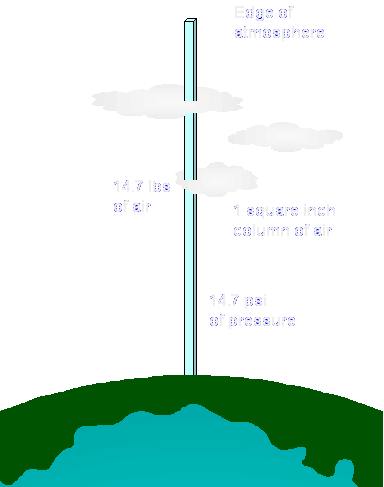
Atmospheric pressure is equal to the weight of a 1 square inch column of air extending all the way to the edge of the earth's atmosphere. At sea level, this column weighs 14.7 pounds, so the atmospheric pressure is 14.7 psi. To make calculations easier, a unit of measurement called "atmospheres" or "ata" is used. 1 atmosphere of pressure is equal to 14.7 psi.
Gauge Pressure
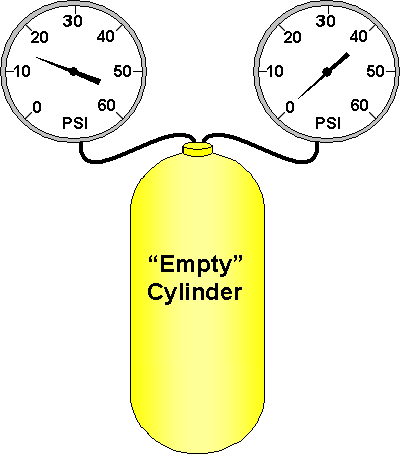
There are times when you are only concerned about pressure beyond atmospheric pressure. For these measurements, we use gauge pressure, which ignores the 14.7 psi of atmospheric pressure that always exists at sea level.
Your submersible pressure gauge is an example of a device that reads gauge pressure. An "empty" cylinder still contains 14.7 psi of pressure at sea level. But your submersible pressure gauge ignores this 14.7 psi of pressure and reads "0" instead.
Your depth gauge is another example. As you'll learn later in this lesson, the pressure you're exposed to while diving is a combination of both atmospheric and water pressure. Your depth gauge is calibrated to read "0" at sea level, therefore ignoring atmospheric pressure and only measuring changes in water pressure.
Absolute Pressure
While diving, both the atmosphere and the water above you exert pressure. The combination of the atmospheric and water pressure is called absolute pressure. And since water is about 800 times denser than air, pressure changes at a faster rate than it does on land.
The rate of pressure increase depends on whether you're diving in salt water or fresh water. Because salt water has more density than fresh water, pressure increases faster as you descend in salt water.
Calculating changes in atmospheric pressure is challenging because air density decreases as altitude increases. Fortunately for us, water doesn't compress like air, so pressure increases at a constant rate with depth.
Absolute Pressure in Salt Water
A 33-foot square inch column of salt water weights exactly 14.7 pounds, and exerts 1 atmosphere of pressure. Therefore, absolute pressure increases by 1 atmosphere every 33 feet.
As the graph to the left illustrates, the pressure at 0 feet is 1 atmosphere. Because 33 feet of salt water exerts 1 atmosphere, the absolute pressure at 33 feet is 2 atmospheres. Pressure continues to increase by 1 atmosphere every 33 feet.
A common mistake divers make when calculating absolute pressure is forgetting the 1 atmosphere of pressure at the surface. Remember, absolute pressure is the combination of pressure from both water AND air.

Absolute Pressure in Fresh Water
Fresh water is less dense than salt water, so the rate of pressure increase is slightly different. Pressure increases by 1 atmosphere every 34 feet, as opposed to 33 feet for salt water.
As the graph to the left illustrates, the pressure at 34 feet of fresh water is 2 atmospheres, 3 atmospheres at 68 feet, and 4 atmospheres at 102 feet. The pressure continues to increase by 1 atmosphere every 34 feet.

Boyle's Law
The volume of an air space changes as the pressure surrounding it changes. The relationship between pressure and volume is best described by Boyle's Law, which states:
"The volume of any gas is inversely proportional to the pressure."
This means that volume decreases as pressure increases, and volume increases as pressure decreases. This law also states that the relationship is proportional. For example, if the pressure doubles, air volume decreases by one half.
Open Air Space Volume, Descent
In the example to the left, an open air space is filled with air and pulled to increasing depths of water. This container is open, so water enters as air volume decreases. This allows the container to retain its original shape and size.
When the bucket is pulled down to a pressure of 2 atmospheres (a depth of 33 feet in salt water) the pressure doubles, so the volume is one half of its volume at the surface.
At 3 atmospheres of pressure (66 feet in salt water) the pressure is 3 times that at the surface, so the air volume is one third of its volume at the surface.
At a pressure of 4 atmospheres, the volume reduces to one quarter of the surface volume. This pattern continues with descent. For example, the volume decreases to one tenth of the surface volume when pulled to 10 atmospheres of pressure.

Open Air Space Volume on Ascent
In this example, the bucket is filled with air at depth, then released to the surface. As it ascends the pressure decreases, which allows the volume to increase.
The bucket is filled at a pressure of 4 atmospheres, or 99 feet of salt water. When the bucket rises to a pressure of 3 atmospheres, the volume increases by 1 third. At 2 atmospheres, the volume is double what it was at 4 atmospheres. And at the surface, the volume is 4 times its original volume.
Since this is an open system, air escapes as it expands. This means the shape and size of the bucket is not affected by the expanding volume of the air on ascent.

Pressure and Closed Air Spaces
Divers are more concerned about the effects of pressure on closed air spaces. These spaces can change in volume or even become damaged as pressure changes.
An example is your wetsuit, which compresses as you descend and expands during ascent. Your body also contains air spaces that can become closed if you are unhealthy or fail to take safety precautions. The next chapter covers these air spaces and how to protect them from pressure-related injuries.
The most severe rate of change in volume occurs from the surface to a depth of about 33 feet. This is because the pressure doubles with just 33 feet of depth. For this reason, you need to be particularly careful with air spaces in shallow water.

Calculating Air Volume Changes
You can calculate exact changes in air volume by using a simple calculation. It is:
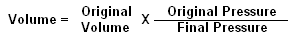
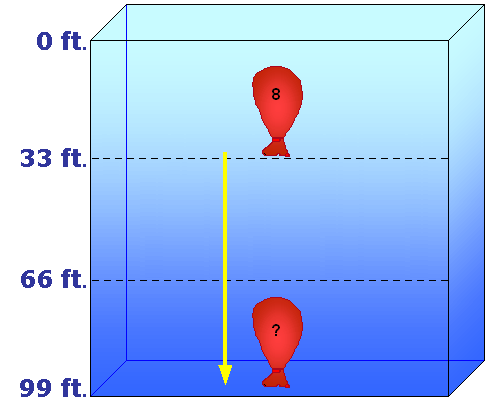
In the example to the left, a balloon contains 8 cubic inches of air at 33 feet. It is then pulled down to 99 feet, and we want to know the new volume.
First, we need to determine the original and final pressures. Since this is salt water, the pressure at 33 feet is 2 ata, and at 99 feet the pressure is 4 ata. Next, we determine the original volume, which is 8 cubic inches. So our formula will look like this:
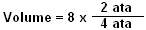
The ratio equals 1/2, and 1/2 of 8 equals 4. So the balloon's new volume at 99 feet would be 4 cubic inches.
Pressure & Air Density
Changes in absolute pressure also affect air density. For example, if the pressure doubles, the volume decreases to one half. Since the same amount of air now occupies half the space, the density is doubled.
Air density is directly proportional to absolute pressure. So if pressure doubles, density doubles as well. The chart to the left illustrates the relationship between pressure, volume, and density.
As the air becomes denser, it doesn't flow as easily through your regulator and body's air passages. This increases breathing resistance, which means you have to work harder to breathe than you do on land. While you'll notice the change in breathing resistance, for most divers it's not a problem unless they exert themselves.

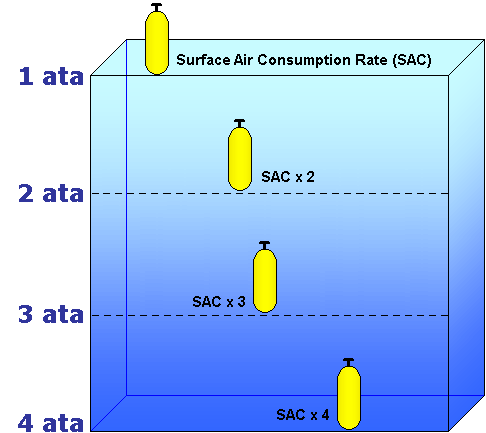
Depth & Air Consumption
There are several factors that influence your air consumption. Physical activity, temperature, psychological comfort, and physical condition are just a few examples. But the most significant factor is your depth, because the air's density increases as your depth increases.
The density of the air determines how long your supply will last. Your air will last half as long at 2 atmospheres of pressure than it does at the surface. Continue down to 3 atmospheres of pressure, and your supply is one third of what it would be at the surface.
For example, if a diver consumes 30 psi per minute at 33 feet of salt water, that same diver will consume 45 psi/min at 66 feet, and 60 psi/min at 99 feet. At the surface, or 1 atmosphere of pressure, the diver would consume 15 psi/min. This is called your surface air consumption rate, and is valuable for predicting your air consumption at planed depths.