The Air Divers Breathe
We tend to take air for granted. After all, it works just fine for us in the environment we live in. There's plenty of it, it keeps us alive, and it doesn't change much as we move from one location to another.
As divers, we need to understand the physical characteristics of air in order to predict changes that occur as we descend in the water. In this lesson, you'll learn the composition of air, its density, and other characteristics that affect us as divers.

The Composition of Air
When many people think of air, oxygen is the first gas that comes to mind. After all, this is the gas that our bodies depend on to survive.
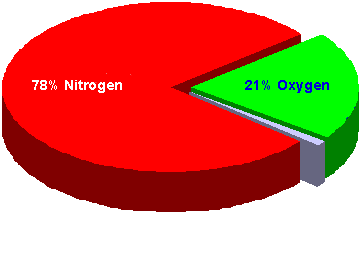
But nitrogen is actually the major component, and makes up about 78% of the air we breathe. Nitrogen is completely inert, so it has no positive or negative effects on our bodies under normal conditions. But as you'll learn later in this course, nitrogen can have negative effects on divers as a result of pressure change.
Oxygen is the gas we depend on for survival, and it makes up about 21% of the air we breathe. Oxygen can also have negative effects on divers, and you'll learn about those effects later in this course.
About 1% of air consists of miscellaneous gases such as carbon dioxide.
The Density of Air
Density refers to a substance's mass per unit of volume. In the United States "pounds per cubic feet" is used to measure density.
The density of air depends largely on altitude and temperature, but for the purposes of this lesson we are mainly concerned with its density at sea level. And at sea level, the density of air is 0.08 lbs/ft³.
Air can be compressed by applying additional pressure, and this increases its density. For example, the density of the air in a full scuba cylinder is much higher than in an empty one. And as you'll learn later in this chapter, air density increases as you descend in the water, and decreases as you ascend.
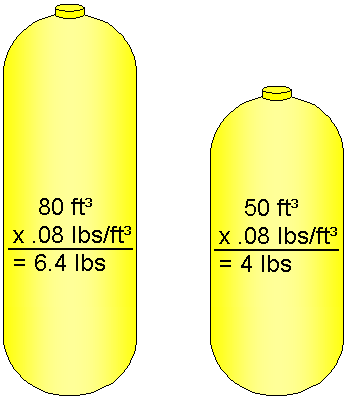
Measuring Scuba Cylinder Air Weight
Air has weight, so our cylinders become lighter as air is consumed. This means a diver who's neutrally buoyant with a full cylinder will become lighter and more buoyant as air is consumed. In order to ensure neutral buoyancy at the end of the dive, extra weight must be added to compensate for the loss of this weight.
To calculate the weight of air in a full cylinder, you multiply the cylinder's capacity by the density of air at sea level. For example, to determine the weight of air in a full 80 ft³ cylinder, multiply 80 ft³ by 0.08 lbs, and you see that the cylinder contains 6.4 lbs of air.
Since you'll be surfacing with air remaining in the cylinder, you'll only need to add 4 or 5 lbs of weight to ensure neutral buoyancy at the end of the dive.